Discovering the Cause of the Ozone Hole
- Catching the World Off Guard
- Ozone and Its Significance
- Solving the Mystery
- Ozone Depleting Compounds
- New Atmospheric Research
- Working for the Common Good
Catching the World Off Guard
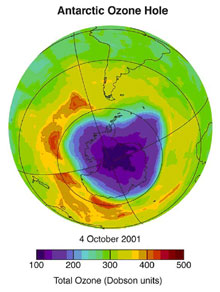
The Antarctic ozone hole forms in the southern hemisphere's spring (September to November) following the bitterly cold and dark Antarctic winter when stratospheric ice clouds promote production of chemically active chlorine and bromine. This, in turn, leads to ozone destruction when sunlight returns in the Antarctic spring. Click image for larger view.
Discovering the Antarctic atmospheric ozone hole was a stunning geophysical surprise of global proportions with potentially disastrous consequences. First announced in 1985 by scientists with the British Antarctic Survey, the large area of depleted ozone at the bottom of the globe aroused a worldwide scurry to understand the phenomenon. Theories abounded. Scientists suggested a host of explanations covering atmosphere dynamics, chemical interactions, and solar physics to decipher the mystery.
Ozone and Its Significance
Ozone is an uncommon but highly important form of oxygen. Most of it is concentrated in a layer of the stratosphere (the upper atmosphere) about 15 to 30 kilometers (a little more than nine to a little less that 19 miles) above the Earth's surface. This ozone layer filters the sun's ultraviolet radiation. Without this protection, humans can suffer over exposure to ultraviolet radiation leading to increased levels of skin cancer, cataracts, and a weakened immune system. Depleted atmospheric ozone can also lead to reduced crop yields, disruptions in the marine food chain, and other harmful effects.
Thinking "Outside the Box" and Solving the Mystery

NOAA scientists launch an ozonesonde via balloon to measure of the vertical profile of the ozone layer. NOAA releases ozonesondes at eight sites worldwide, including the Amundsen-Scott South Pole Station. It also uses satellite and ground-based systems to continuously monitor stratospheric ozone. Click image for larger view.
NOAA scientists were at the forefront of the international discussions and scientific endeavors to find the cause of the ozone hole. In a landmark paper published in the journal Nature in 1986, NOAA scientist Dr. Susan Solomon and colleagues offered a theory: Human-produced chlorine compounds could be interacting with stratospheric ice clouds and, in the unique meteorological setting of the polar regions, this interaction could produce extreme ozone losses. It was a remarkable insight and scientific breakthrough, one that required thinking "outside the box" of any one discipline in the atmospheric sciences. Chemistry, dynamic interactions between gases and airborne particles, and seasonal meteorology were all critical aspects of the theory.
And as it turned out, Solomon and her colleagues were right. They played key roles in experimental expeditions to Antarctica that provided the evidence. Together with colleagues from the international scientific community, NOAA scientists, led by Dr. Solomon, embarked on the National Ozone Expeditions of 1986 and 1987. Solomon, David Hofmann, Ryan Sanders, Art Schmeltekopf, George Mount, and others made key measurements of chlorine-containing gases, ozone, and other trace gases during those Antarctic campaigns. The measurements confirmed that the theory advanced in the 1986 Nature paper was the only explanation that "fit" the observations.
Reducing Ozone Depleting Compounds

NOAA atmospheric scientist Susan Solomon theorized that human-produced chlorine compounds could be interacting with stratospheric ice clouds in the unique meteorological condition of the Antarctic.. Click image for larger view.
Following the discovery of the Antarctic ozone hole and after NOAA research explained its cause, governments around the world recognized the need to reduce the production and consumption of a number of chlorofluorocarbons and halons. These were the man-made compounds that were depleting atmospheric ozone. This resulted in nations agreeing to the Montreal Protocol on Substances that Deplete the Ozone Layer on September 16, 1987. The Protocol became effective on January 1, 1989, following ratification by 29 countries and the European Economic Community. Since then over 180 countries have ratified it.
Launching a New Area of Atmospheric Research
The work by NOAA scientists and their colleagues on this issue launched a new area of research in atmospheric science–the study of gas-particle reactions. Prior to the discovery of the ozone hole, this so-called "heterogeneous chemistry" was a fledgling area of stratospheric research (a fact that explains why the ozone hole caught the entire research community off guard!). Today, the field is transformed and heterogeneous chemistry is recognized as a pivotal factor in ozone depletion as well as other atmospheric issues.
Working for the Common Good

Antarctic stratospheric clouds like those shown here are where Dr. Solomon and her colleagues theorized and later etablished that chemical interactions with human-produced chlorine and bromine compounds could destroy ozone. Click image for larger view.
The discovery that human-produced chlorine and bromine compounds were indeed the cause of the ozone hole provided the scientific underpinning for nations worldwide to strengthen protections for the Earth's ozone layer under the Montreal Protocol. Because of those actions, atmospheric scientists expect that by about the middle of the 21st century, man-made chlorine and bromine compounds will no longer deplete planet's the ozone layer. This stands as an outstanding example of NOAA science working at a global scale for the common good of humankind.